Which Elements Have An Anomalous Electron Configuration
Third Transition or 5d-series. Electron of gp 16 element 6 V.

So Called Anomalous Electron Configurations Aren T Openstax Chemistry 2e 6 4 Youtube
Chemical Properties of Group 18 Elements.
Which elements have an anomalous electron configuration. These elements require one electron to finish their octet. These elements are also called representative elements. Xii Electron affinity Due to the presence of stable electronic configuration they have no tendency to accept additional electron.
There are over 20 elements that do not follow the building up principle. Nuclear-electron and electron-electron forces are attributed factors. They readily lose electron to give monovalent M ions.
An example is beryllium to boron with electron configuration 1s 2 2s 2 2p 1. Electron of gp 17 element 7. The configuration for Pb can be written as Xe6s 2 4f 14 5d 10 6p 2.
Alkali metals have a corresponding Noble gas ns 1 electronic configuration. They can complete their octet either by picking up an electron or sharing an electron. For example using the building up principle Cr would have an electron configuration of Ar4s 2 3d 4.
The noble gases are inert in nature because of their completely filled subshells. 5d-orbitals are gradually filled up. Electron of gp 15 element 5 V.
Electron of gp I element 1 V. Electron of gp 14 element 4 V. Hence they are never found in free state in.
Cr Ar 4s1 3d5 Cu Ar 4s1 3d10 To. The general electronic configuration of group 17 is. An alkali metal easily loses one electron to leave an octet or pseudo-noble gas configuration so those elements have only small values for IE.
Electron of gp 13 element 3 V. They occupy the first column of the periodic table. The electron-shell configuration of elements beyond hassium has not yet been empirically verified but they are expected to follow Madelungs rule without exceptions until element 120.
Transitioning to a new period. Alkali elements are LithiumLi SodiumNa Potassium K Rubidium Ru Cesium Cs and Francium Fr occupying successive periods from first to seven. The third period elements of the periodic table are known as typical elements because they dont show anomalous properties with their respective groups.
The loosely held s-electron in the outermost valence shell of these elements makes them the most electropositive metals. Solution is paramagnetic and has electrical conductivity due to presence of unpaired electron in the cavities of ammonical solution and ammoniated cations and electrons respectively. The shorthand electron configuration for Fe is Ar4s 2 3d 6.
The oxidation states of all the elements belonging to this group are -1. Element 121 should have the anomalous configuration 8s 2 5g 0 6f 0 7d 0 8p 1 having a p rather than a g electron. 1011 Electronic Configuration All the alkali metals have one valence electron ns1 Table 101 outside the noble gas core.
Electronic configuration of H is similar to alkali metal as both have 1 valence electron. Moving from the s-block to the p-block. A p-orbital loses an electron more easily.
These are sodium magnesium aluminium silicon phosphorus sulfur chlorine and argon. Elements marked with an asterisk have anomalous configurations. Electron of gp 2 element 2 V.
This series consists of elements from LaS7 to Hg80 except 14 elements of lanthanide series from CeS8 to Lu71. Using the Aufbau principle you would write the following electron configurations Cr Ar 4s2 3d4 Cu Ar 4s2 3d9 The actual electron configurations are. Francium is a radioactive element with very low half-life.
Some elements do not follow the Aufbau principle there are some alternate ways that electrons can arrange themselves that give these elements better stability. Therefore electron affinity is almost zero. M M electron M xNH 3 MNH 3 x Ammoniated metal ion Electron yNH 3-- eNH 3 y- Ammoniated electron Colour of such solution is blue.
All the elements of group 17 have 7 electrons in its valence shell.

So Called Anomalous Electron Configurations Aren T Openstax Chemistry 2e 6 4 Youtube
Solved What Are The 21 Anomalous Elements Of The Periodic Chegg Com

Electron Configuration And Periodicity Ppt Download

Anomalous Configuration Of Chromium And Related Issues Anomalous Configuration Of Chromium

Anomalous Configurations Such As Cr And Cu Viper

Number Of Unpaired Valence Electrons Green Is Anomalous Electron Download Scientific Diagram

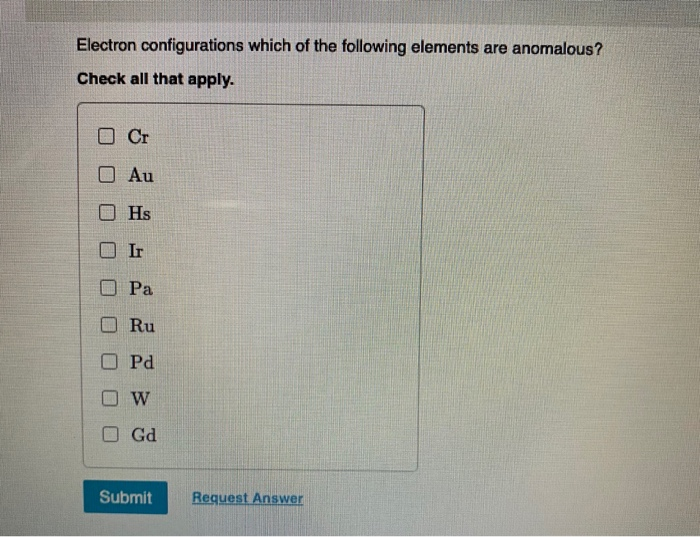
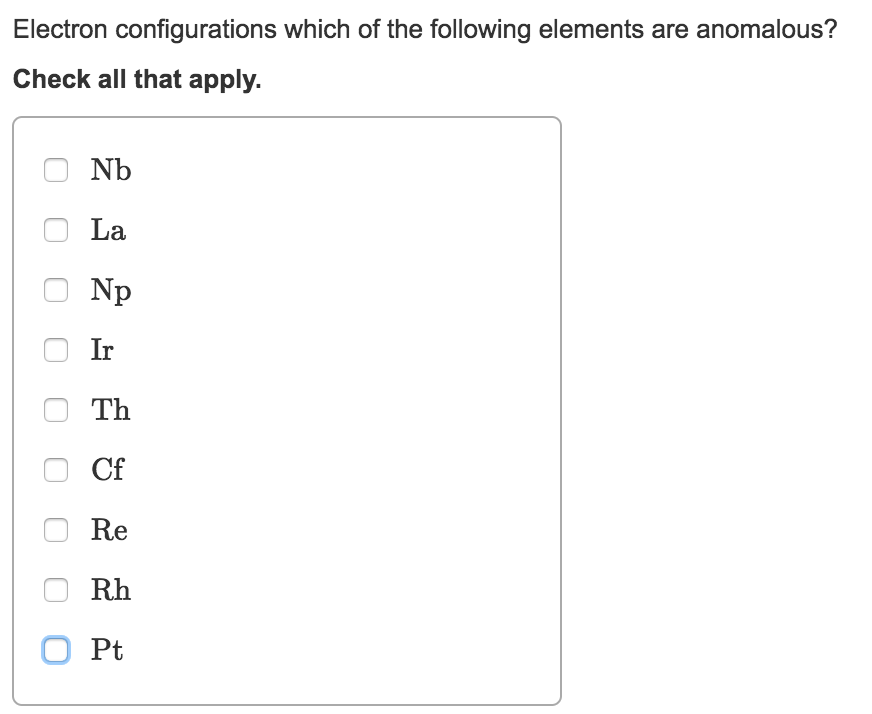
Solved Electron Configurations Which Of The Following Chegg Com

Inorganic Chemistry Anomalous Oxidation States Of Transition Metals Chemistry Stack Exchange
Electron Configurations And Orbital Box Diagrams Pathways To Chemistry
Belum ada Komentar untuk "Which Elements Have An Anomalous Electron Configuration"
Posting Komentar